

The anomeric effect is synthetically important as it is being an important part of in Koenigs-Knorr glycosidation, the oldest glycosylation reaction. The magnitude of this anomeric effect is quantitated to be about 1.4 kcal/mol for OCO stabilization systems. The anomeric effect or Edward Lemieux effect is the stereoelectronic effect describing the preference of axial orientation on the anomeric carbon, while the equatorial one is supposed to be the preferred one being less hindered. It can also be understood as the opposite side to that of CH 2OH.Īn easy way to remember this nomenclature is that alpha(α) goes for axial, (a→a), while beta(β) goes for equatorial, (b→e). On the other hand, the beta position indicates the opposite or equatorial position of anomeric and C-4 hydroxyl groups. Some literature suggests that it should be regarded as the same side as CH 2OH. Alpha indicates the position of hydroxyl group (attached to anomeric carbon) to be in the same or axial direction as that of hydroxyl fo C-4. Nomenclature of anomersĪnomers are generally named by prefixes of alpha (α) or beta (β). This leads the equilibrium mixture of α-D glucose and β-D glucose to be in a ratio of 9:16 with a minute quantity of acyclic aldehyde form, as shown above. For example, due to more stable equatorial positions of hydroxyl groups and less strained chair conformation, β-D glucose is more stable than α-D glucose. The anomerization equilibrium shifts to one side if there is extra stability seen in one of the anomers. It is a reversible reaction mechanism that typically leads to an equilibrium mixture of two anomeric forms of a carbohydrate, whichever may be the starting one. For reducing sugars, this anomerization is in fact mutarotation. It is a spontaneous process that may sometimes involve acidic or basic catalysts.
#NON REDUCING ANOMERIC CARBON FREE#
In other words, the free hydroxyl forms (as in hemiacetals) make reducing sugars, while occupied hydroxyls (as in acetals) make non-reducing sugars. If that oxygen is free, the sugar turns out to be a reducing one, and if it is bound to any further structure, it will be a non-reducing sugar. It can be concluded here that, it is the oxygen atom present on anomeric carbon, which determines whether a sugar structure is a reducing or not.
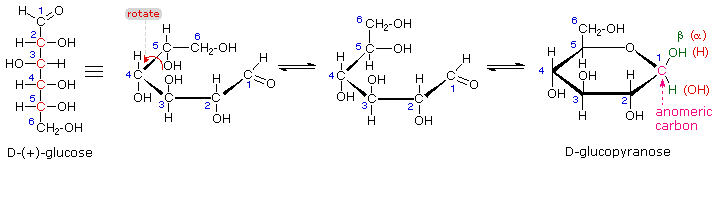
Except for the anomeric hydroxyl, all other such hydroxyls are not involved in determining whether a sugar is reducing one or not. Reducing sugars can therefore react with oxidizing agents, like Benedict’s solution. If that specific hydroxyl is not attached to any other structure, that sugar is a reducing sugar. The anomeric carbon, or to be very specific, the aldehydic carbon in aldoses and ketonic carbon in ketoses, has a hydroxyl group, which can be termed as anomeric hydroxyl. On the other hand, cellulose, a polysaccharide in plants, is synthesized from β-D glucopyranose. For example, glycogen, a polysaccharide of glucose in animals is synthesized from α-D glucopyranose. This specificity leads to specific products in certain conditions. They are found as an equilibrium mixture upon dissolution.Įnzymes, due to their specificity, are best suited for the identification of alpha and beta sugars or to differ between between the two. These cyclic anomers of sugars are in equilibrium with each other in solution form and spontaneously interconvert by the process of mutarotation.įor example, α-D glucose and β-D glucose are anomers to each other. The resultant structures are designated as alpha(α) or beta(β) carbohydrates. Based on these possibilities, that specific carbon is called the anomeric carbon, and also the stereogenic center.ĭuring the ring formation of carbohydrates ( Aldose and Ketose sugars), at C-1 of aldoses and C-2 of ketoses, an anomeric carbon center is formed. There exist two possibilities of the orientation of the hydroxyl group present on such carbon centers. In carbohydrates, these stereoisomers are created by intermolecular cyclization through acetal or ketal groups. The anomeric center is also termed as the stereogenic center in stereoisomers. Identification of Alpha and Beta Sugars.


 0 kommentar(er)
0 kommentar(er)
